Open Targets Platform 24.06 has been released!
The latest release of the Platform — 24.06 — is now available at platform.opentargets.org.
Key points
In addition to our regular data updates:
- Users can now customise the associations view to explore evidence for a set list of entities
- We have updated our gene burden dataset with version 5 of the AstraZeneca PheWAS Portal and added new evidence for targets associated with prostate cancer
- Notable data updates include ChEMBL 34 and the Gene2Phenotype skeletal panel
Key stats
| Metric | Count |
|---|---|
| Targets | 63,226 |
| Diseases | 28,198 |
| Drugs | 18,041 |
| Evidence | 17,703,456 |
| Associations | 8,079,215 |
Additional metrics are available on the Open Targets Community.
View associations for a custom list of entities
Our Associations on the Fly view in all target and disease associations pages now allows you to upload a custom list of entities to obtain a tailored associations view. A much requested feature, this will allow users to easily view target-disease evidence for the specific entities they are interested in, and facilitate therapeutic hypothesis building.
Users can upload .txt, .csv, .tsv, .xlsx, and .json files with up to 2,000 rows. The input file data is validated and matched to the entity names in the Platform, so that users can verify the specific results they want displayed.
Once selected, the entities will appear as pinned rows in the Associations on the Fly view. These can be unpinned as users refine their list.
More information on this feature is available in the documentation.
Gene Burden data update
AstraZeneca PheWAS Portal
We have added new data to our Gene Burden widget, which incorporates the results from the aggregation of rare and ultra-rare variants at the gene-level.
In particular, we updated the gene burden results from AstraZeneca’s PheWAS Portal, integrating the version 5 release which aggregates the ExWAS results from a cohort of 470,000 individuals, and contains a more robust and better-powered collapsing analysis derived from the UK Biobank. We have also added links to the respective AstraZeneca PheWAS Portal pages.
Thanks to this update, we integrate an additional 9,533 evidence from the PheWAS Portal, for a total of 21,300, practically doubling the number of associations.
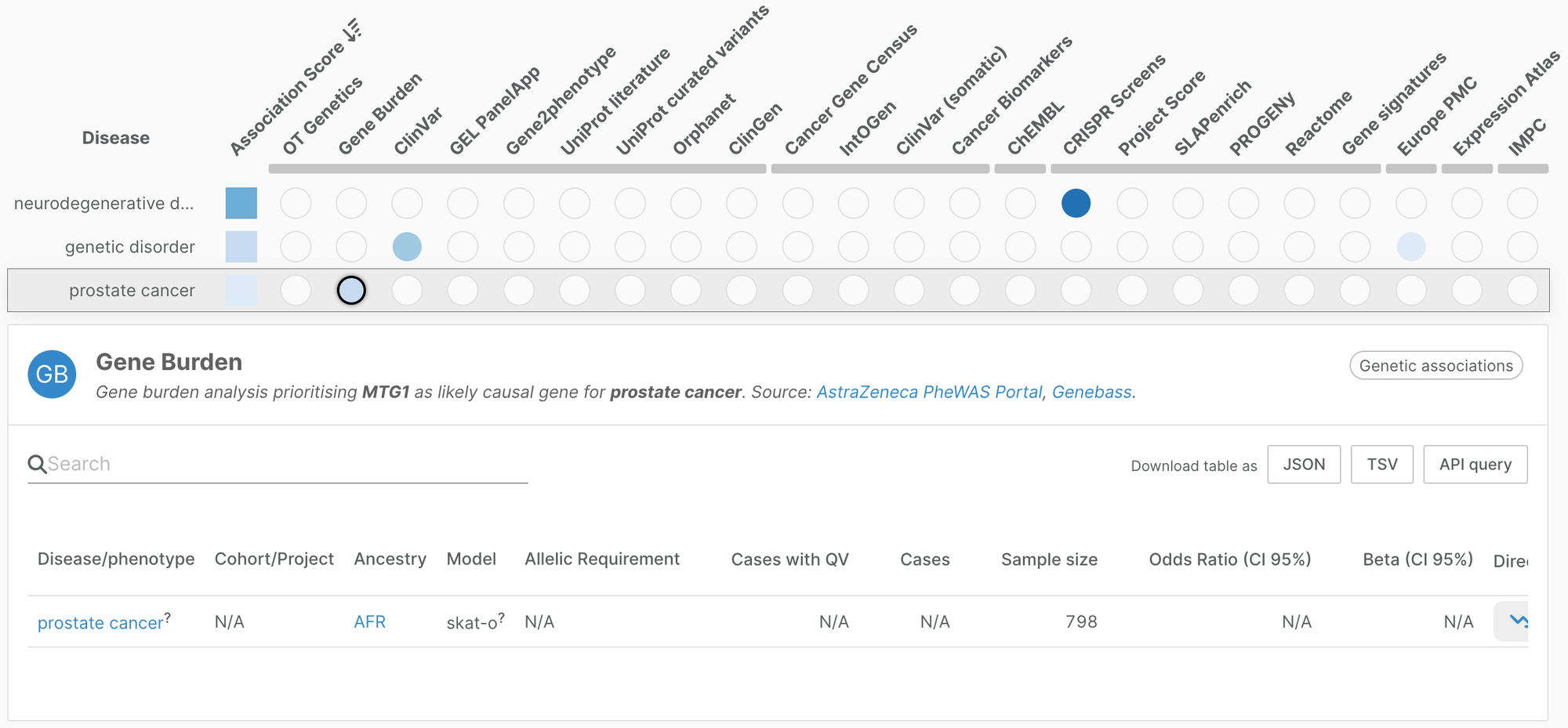
Gene burden evidence for prostate cancer
In addition, we have added ancestry-specific gene burden evidence for prostate cancer. Soh et al. used rare coding variants from whole exome sequencing of 798 Black South African men to identify genes significantly associated with prostate cancer.
The study highlighted three genes MTG1, H3C1, MBP for which little is known about their role in prostate cancer.
Soh, P.X.Y., Mmekwa, N., Petersen, D.C. et al. Prostate cancer genetic risk and associated aggressive disease in men of African ancestry. Nat Commun 14, 8037 (2023).
Update to SCHEMA consortium data
We have expanded the whole exome sequencing data previously integrated from the SCHEMA consortium. In addition to the 10 genes associated with schizophrenia as a result of a meta-analysis, four genes — TRIO, XPO7, HERC1 and SETD1A — showed statistical significance with high pathogenicity score (class I) variants, and we are therefore able to provide direction of effect assessments.
Singh, T., Poterba, T., Curtis, D. et al. Rare coding variants in ten genes confer substantial risk for schizophrenia. Nature 604, 509–516 (2022).
Other updates
Following community feedback, we have removed categorical burden tests from the data we integrate from Genebass.
Data updates
This release includes a number of data updates, including ChEMBL, Gene2Phenotype, Genomics England PanelApp, COSMIC, EVA, Probes and Drugs.
ChEMBL 34
Released in April, ChEMBL 34 integrates, for the first time, drug approvals from the European Medicines Agency. This includes 882 drugs prior to January 2023, some of which are only authorised by EMA, and therefore gives a more complete picture of drugs on the market.
Additionally, ChEMBL now distinguishes antibody-drug conjugates (ADCs) as a separate modality which are now represented in the Open Targets Platform.
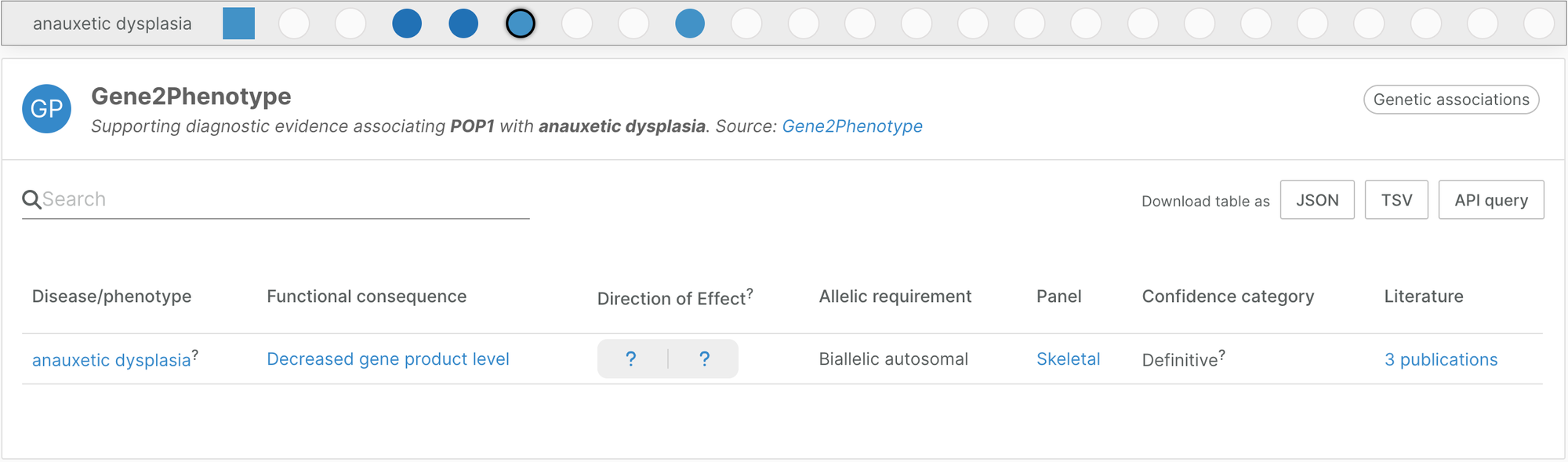
Gene2Phenotype skeletal panel
We introduced a new Gene2Phenotype panel in this release, adding evidence for rare disease genetics. It contributes 328 new disease/target associations between 289 diseases with musculo-skeletal implications and 260 unique targets.
Gene2Phenotype provides high quality curated genetic associations based on literature. Panels are reviewed by consultant clinical geneticists. The skeletal panel is maintained by Andreas Zankl, Professor of Medical Genetics and Genetic Medicine, University of Sydney.
Genomics England PanelApp
The updated PanelApp data from Genomics England provides almost 2,500 more disease target evidence and 13 new disease related gene panels including a panel for acute rhabdomyolysis and a panel for Autoinflammatory disorders.
As usual, please share any comments, questions, or suggestions on the Open Targets Community.